[ad_1]
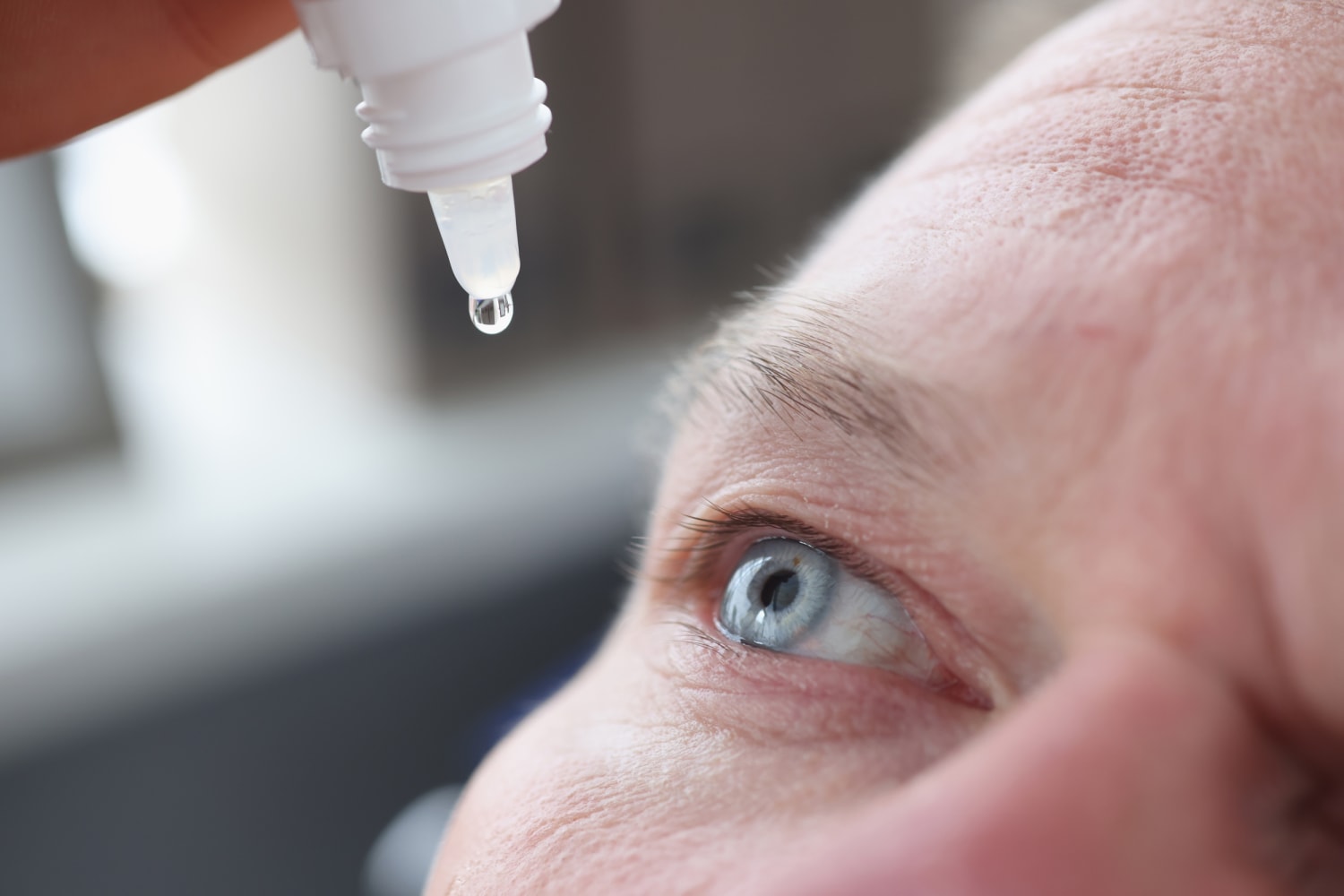
One person has died and at least three others are left with permanent vision loss because of a bacterial infection possibly linked to a brand of over-the-counter eyedrops, according to the Centers for Disease Control and Prevention.
A majority of those affected reported using preservative-free EzriCare Artificial Tears before becoming ill, the CDC reported in a statement dated Jan. 20.
While the infections have not been definitively traced to the eyedrops, the CDC recommended that “patients immediately discontinue the use of EzriCare Artificial Tears until the epidemiological investigation and laboratory analyses are complete.”
So far, the CDC team has identified at least 50 people in 11 states with Pseudomonas aeruginosa, a type of bacterium resistant to most antibiotics. Cases have been reported in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah and Washington.
Most patients said they’d used EzriCare Artificial Tears before becoming ill.
Eleven developed eye infections, at least three of whom were blinded in one eye. Others had respiratory infections or urinary tract infections. One person died when the bacterium entered the patient’s bloodstream.
It is unclear whether the affected patients had underlying eye conditions, such as glaucoma or cataracts, that would have made them more susceptible. Symptoms of an eye infection include pain, swelling, discharge, redness, blurry vision, sensitivity to light and the feeling of some kind of foreign object stuck in the eye.
Pseudomonas aeruginosa bacteria are commonly found in water and soil and even on the hands of otherwise healthy people. Infections usually occur in hospital settings among people with weakened immune systems.
This type of bacterium is often resistant to standard antibiotics.
“That’s what’s so concerning,” said Dr. Jill Weatherhead, an assistant professor of tropical medicine and infectious diseases at the Baylor College of Medicine in Houston. “Our standard treatments are no longer available” to treat this infection.
The drops under investigation are labeled as preservative-free. That is, the product does not contain anything that might prevent microbiological growth. The product could have been contaminated during the manufacturing process or when a person with the bacteria on his or her skin opened the container.
The CDC found the bacteria in bottles of the eyedrops and is testing to see whether that bacteria matches the strain found in patients.
As of Tuesday, EzriCare Artificial Tears had not been recalled. They have been sold on Amazon and at stores such as Walmart.
[ad_2]
Source link
