[ad_1]
MET ablation affects cytoskeleton organization and nuclear 3D architecture
LoVo cells encode for an aberrant uncleaved MET receptor of 190 kDa constitutively phosphorylated independently from the ligand14. This alteration occurs at a post-translational level, and MET gene is neither mutated nor amplified in these cells14. Thus, LoVo cell line represents a unique and ideal model to study MET-driven phenotypical alterations in cancer cells. By means of CRISPR/Cas9 technology15, we performed a MET gene KO. Successful MET ablation was confirmed by protein analyses both by western blot (Fig. 1a) and immunofluorescence (Fig. 1b). In line, the activation of MET downstream effectors, AKT and ERK appeared downregulated in MET-KO cells (Fig. 1a). Furthermore, MET-KO ablation reduced cell proliferation rate (Fig. 1c) and triggered an impressive modification in the cell phenotype: indeed, the typical fibroblastic-like feature of MET aberrant cells was clearly reverted upon ablation, turning the cells into a squamous epithelial shape with enhanced cell boundaries (Fig. 1d). The Ptychographic quantitative phase imaging technology of Phasefocus LivecyteTM system allowed to precisely assess in living cells several morphometric parameters by measuring the main phenotypical changes derived from MET silencing. First, we detected a statistically significant increment of the cellular area and the perimeter (Fig. 1e, f) in MET-KO compared to MET aberrant (MET + ) cells. In addition, the morphological differences triggered by MET ablation were confirmed by a remarkable increase in length-width ratio (Fig. 1g), suggesting an elongated cell shape associated with a strong reduction in cells’ sphericity (Fig. 1h). Prompted by the remarkable morphological changes observed, we further investigated the actin microfilaments organization of the MET-KO cells in comparison with the control cells, where MET signaling is constitutively activated. Using both 2D standard immunofluorescence (Fig. 2a) and 3D reconstruction from confocal imaging (Fig. 2b), we recorded a precise orientation of the actin cytoskeleton. In detail, the 3D rendering revealed that MET-activated cells bear aberrant actin structures, located close to the nucleus, associated with a well-defined rounded nuclear architecture, and distributed over the entire cell height (Fig. 2a, b, white arrows). In normal conditions, nuclei are usually covered and stretched by bundles of actomyosin, which connects the nuclear envelope to the cytoskeleton. In MET+ cells, the derailed actin fibers alignment leads to the coalescing of the fibers in the apical plane, by forming actin structures, to which we refer to as actin-patches. Notably, MET KO cells reverted to a quasi-normal actin filament fibers configuration (Fig. 2a–c) with the actin-patches structures completely disappearing, while remaining stable over time in aberrant MET+ cells (Fig. 2c). Interestingly, the nuclear height impressively decreased upon MET ablation, and these changes were evident already after 24 h from seeding (Fig. 2d). On average, after 48 h the nuclear height measured about 12 μm in MET+ positive cells, while in KO the average height was about 8 μm (Fig. 2d and quantification). Through the Operetta CLS High-content screening system we performed further quantitative analyses on a large number of cells. MET-KO cells despite having increased nuclear volume and surface area (Supplementary Fig. 1a, b), displayed decreased nucleus sphericity and height (Supplementary Fig. 1c, d), with a markedly prominent nucleus footprint area (Supplementary Fig. 1e) consistent with a flat squamous morphology. Aiming to confirm these observations in additional MET+ dependent cellular models, we decided to evaluate actin cytoskeleton morphology in human gastric carcinoma GTL16 cells, characterized by MET activation due to > 10 fold MET gene amplification and overexpression, in absence of activating mutations16. Intriguingly, around 20% of analyzed cells showed actin aberrations like the actin patches detected in LoVo cell line (Supplementary Fig. 2a, b – white arrows), associated with spherical nuclei (Supplementary Fig. 2c). We further employed ultrastructural imaging by transmission electron microscopy (TEM) to visualize the aberrant actin patches in MET+ cells. Intriguingly, TEM analysis highlighted abnormal groups of internalized microvilli-like structures (Fig. 2e, detail on the right) collected inside rounded and disorganized actin-based thick bundles (Fig. 2e, left panel, white arrows). We propose that the perinuclear microfilaments, by coalescing at the cell surface, could induce a global invagination of the cell membrane and consequent internalization of the apical microvilli. Next, we set to confirm by live-cell imaging that these actin patches were not caused by artifactual fixation of actin bundles. We employed a retroviral vector encoding for the LifeAct peptide, which is able to stain actin microfilaments in living cells without impairing actin polymerization17, conjugated with mCherry fluorophore. We monitored overtime the actin dynamics of MET aberrant cells stably expressing mCherry-LifeAct. As reported in Supplementary videos 1 and 2, LoVo-LifeAct replicated the actin-patches aberrations observed in fixed cells, and by monitoring them over time, we recorded structures highly dynamic and continuously rotating around the nucleus (Supplementary Fig. 3a, Supplementary videos 1 and 2). Better resolved images of the aberrant actin patches were obtained in label-free conditions by means of Optical Diffraction Tomography (ODT), which detects variations between the refractory indexes (RI) of different cell compartments. With ODT imaging, the actin patches appeared as circular structures with a higher RI on the edges and a lower RI inside, where a jagged and reticular pattern suggests the presence of chaotic actin filaments (Fig. 2f, Supplementary Fig. 3b). The overlap between LifeAct and Phalloidin signal with ODT detection was confirmed in fixed cells as reported in Supplementary Fig. 3c, d (yellow arrows). Moreover, in living MET+ aberrant cells ODT time-lapse imaging recorded the progressive development of the actin patches, that reached the mature structure after 12 h (Fig. 2g, Supplementary video 3). Intriguingly, during cell division, actin-patches showed asymmetric distribution and only one of the two daughter cells inherits the aberrant actin-patches (Supplementary video 4). Finally, the absence of similar actin abnormalities in the MET-KO model was validated also with ODT technology (Supplementary video 5). Overall, ODT and LifeAct staining confirmed the presence of an abnormal and steadily moving actin-based formation, namely actin-patches, in cells with MET+ sustained activation. These structures maintain a physical connection with the nuclear envelope, with a consequent asymmetric confinement during cell division.
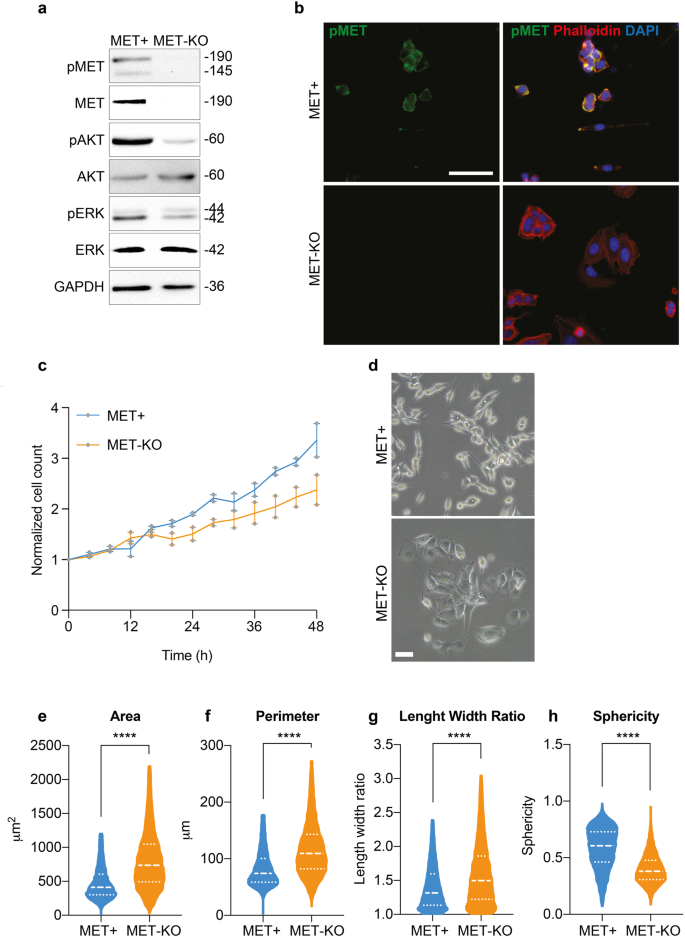
a Protein analysis of MET signaling pathway in LoVo control (MET + ) and LoVo MET-KO cells. GAPDH was used as loading control. b LoVo MET+ and MET-KO stained with pMET (green) and Phalloidin (red) and counterstained with DAPI (blue). Scale bar: 50 μm. c Evaluation of cell proliferation over a time-lapse of 48 h in LoVo MET+ and MET-KO by means of Phasefocus LiveCyteTM platform. Pictures were taken every 4 h. Data were normalized on cell count at T0 and shown as mean ± SEM between two independent experiments. A total of 800 cells was analyzed in each experiment. d LoVo MET+ and MET-KO morphology visualized with phase contrast microscopy. Scale bar: 100 μm. e–h Measurement of cell area, perimeter, length/width ratio and sphericity of LoVo MET+ and MET-KO in a 48 h time-lapse performed with Phasefocus LiveCyteTM. Pictures were taken every 4 h. Median and quartiles distribution are plotted. Outliers were identified and cleaned from results by means of ROUT method (Q = 1%). A total of 800 cells was analyzed. Statistic was calculated by T-test.
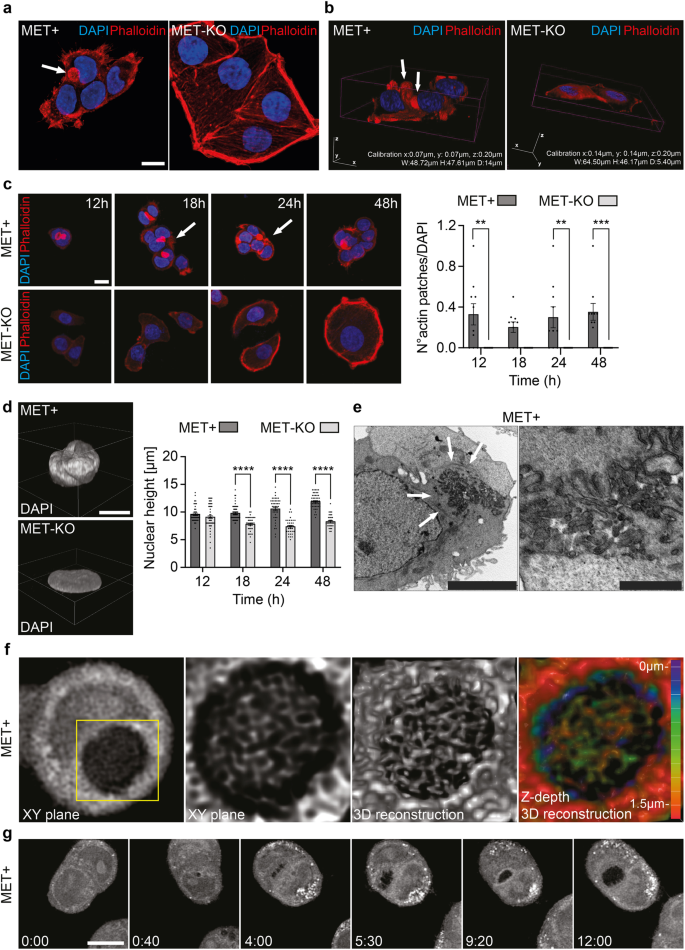
a Actin cytoskeleton and nucleus visualization in LoVo MET + /MET-KO fixed cells through Phalloidin (red) and DAPI (blue) staining. White arrows indicate the actin patches observed in MET+ cells. Scale bar: 20 μm. b Confocal 3D rendering of cells fixed and stained as in (a). White arrows indicate the actin patches observed in MET+ cells. c Nucleus and actin visualization in LoVo MET + /MET-KO cells fixed after 12/18/24/48 h from seeding. White arrows points at the actin patches. A quantification of the number of actin patches observed per field and normalized on DAPI is provided for each time point. 10 fields per condition were analyzed (181 and 135 cells for MET+ and MET-KO samples respectively). Data are reported as mean ± SEM. Statistic was calculated by Two-way ANOVA. Scale bar: 20 μm d Confocal 3D rendering of representative nuclei from experiment in (c). Nuclear height quantification was performed on 10 fields for each time point as in (c) by analyzing Z-stacks images. Data are shown as mean ± SEM. Statistic was calculated by Two-way ANOVA. Scale bar: 20 μm. The scale bar refers to the xy-plane. e Transmission electron microscopy of LoVo MET+ cells. White arrows point at actin filaments. Scale bars: left 5 μm, right 1 μm. f ODT and 3D reconstruction of LoVo MET+ aberrant actin patches. Z-depth coding is reported as a scale of colors. g ODT time lapse of LoVo MET+ cells showing actin patches generation. Pictures of cells were acquired every 10’ for 12 h. Full video is provided as Supplementary video 3. Scale bar: 10 μm.
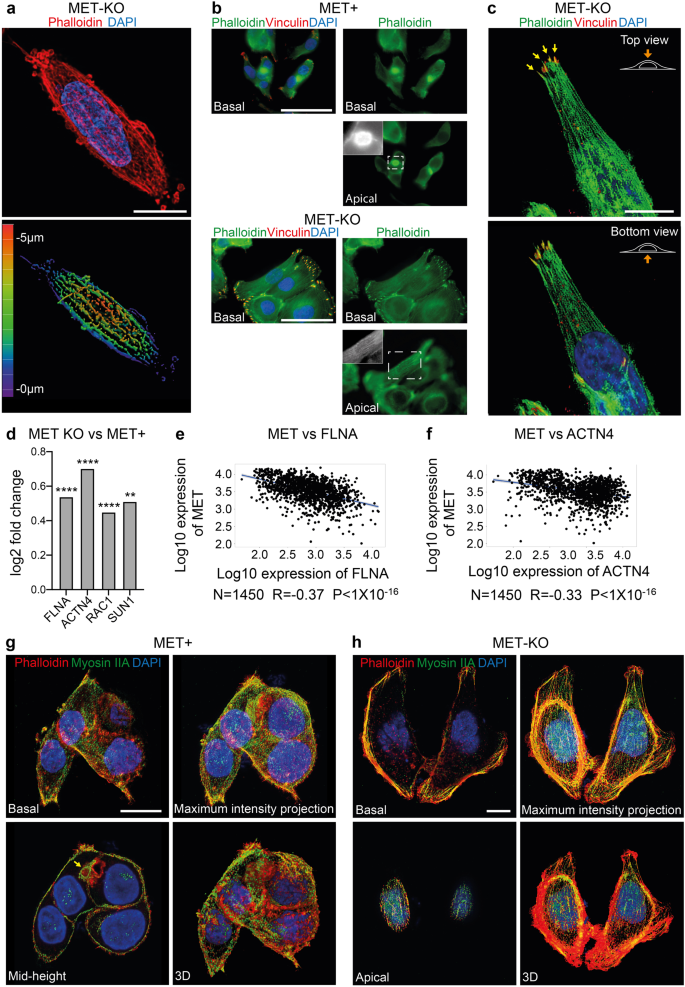
a Super resolution imaging of LoVo MET-KO cells stained with Phalloidin (red) and DAPI (blue) performed with X-Light V3 – DeepSIM microscope. Z-depth coding is reported as a scale of colors in the lower panel. Scale bar: 10 μm. b Focal adhesions (red) and actin cytoskeleton (green) visualization through Vinculin antibodies and Phalloidin staining in LoVo MET-KO and MET+ cells. Nuclei were stained with DAPI (blue). Pictures of basal and apical plane of cells are provided. Scale bar: 50 μm c Top and bottom view of a MET-KO cell stained with Phalloidin (green), Vinculin (red) and DAPI (blue) and visualized in 3D reconstruction. Yellow arrows point at the Actin Cap Associated Focal Adhesions (ACAFAs). Scale bar: 10 μm. The scale bar refers to the xy-plane. d Log2 fold change of filamin A (FLNA), actinin 4 (ACTN4), Rac Family Small GTPase 1 (RAC1) and Sad1 and UNC84 Domain Containing 1 (SUN1) expression in LoVo MET-KO cells compared to LoVo MET+ cells according to RNAseq data. ****Padj < 0.0001 **Padj < 0.01. e, f Pearson correlation between the expression of MET and FLNA (e) or ACTN4 (f) in colorectal cancer according to gene arrays data available on GEO. R and P-value are reported. g, h Non-muscle myosin IIA (green), actin cytoskeleton (Phalloidin – red) and nuclei (DAPI – blue) staining in MET-KO and MET+ cells. Pictures from different planes of section, as well as maximum intensity projection and 3D rendering are provided. Scale bar: 10 μm.
MET inactivation is required for the appropriate perinuclear actin cap assembly
The cytoskeletal tensions provided by the aligned perinuclear actin cap in interphase cells have been reported to compress the nucleus and to maintain a flat phenotype4. Therefore, we hypothesized that MET-driven alterations may associate to a misalignment of the perinuclear actin fibers, resulting in increased nuclear height. First, using a super-resolution microscope, we were able to observe the restored alignment of the actin cap fibers, in MET-KO cells (Fig. 3a). These fibers now cover the entire nuclear height on the apical plane, as appreciable in the 3D rendering of the phalloidin staining in Fig. 3a. Next, we applied vinculin staining to detect the terminal edges of the actin fibers, ending into the focal adhesions (FAs). MET+ aberrant cells displayed poorly organized FAs, most likely due to the lack of a proper alignment of the actin fibers, which fail to reach the periphery of the cells and bind vinculin adhesion protein (Fig. 3b). On the contrary, MET ablation restored the actin microfilaments, with properly aligned actin bundles detected in the apical plane (Fig. 3b and black and white insert). Also, a remarkably well organization of the FAs was easily detectable by immunofluorescence in the basal plane of KO cells. Indeed, the single fibers of the cap cover the apical plane by ending at the cellular edge, where they bind with the actin cap-associated focal adhesion (ACAFAs) (arrows in Fig. 3c). Consistently, genes of the actin cap superfamily, encoding for structural proteins such as filamin A (FLNA) and alpha-actinin 4 (ACTN4), together with the master regulator of actin cytoskeleton reorganization (RAC1), and the LINC complex protein SUN1 appeared upregulated in MET KO compared to MET positive cells (Fig. 3d). So far, these results hint that MET activation may represent a negative regulator for the perinuclear actin cap genes. Following this rationale, we set to interrogate a dataset of gene array data collected from 1450 CRC patients18. Interestingly, we outlined a significant negative correlation between the amount of MET and the expression of both FLNA and ACTN4 (R = − 0.37 and −0.33 respectively) (Fig. 3e, f). The functionality of the perinuclear actin stress fibers is ensured by the association with non-muscle myosin IIA units, which confer contractility to the apical fibers4. In MET+ aberrant cells the actin-rich patches appeared surrounded by myosin IIA, which formed a ring myosin decorated structure, likely resulting from an altered organization of the actin cap bundles, which collapsed into these spots enriched for actin (Fig. 3g mid-height). On the contrary, in KO cells the full-length of the perinuclear actin filaments was associated with myosin IIA, indicating a fully functional contractile actin cap (Fig. 3h). To further prove that MET is the main mediator of actin cap cytoskeletal changes, we performed a rescue experiment, which restored the activation of MET by introducing a fusion protein of constitutively active MET, namely TPR-MET19 (Supplementary Fig. 4a). MET rescue strikingly returned the phenotype of LoVo cells (Supplementary Fig. 4b), resulting in a boosted proliferation rate (Supplementary Fig. 4c), decreased cell area (Supplementary Fig. 4d) and perimeter (Supplementary Fig. 4e). Most importantly, we recorded an increased vertical expansion of the cells, quantified as cell sphericity (Supplementary Fig. 4f) and thickness (Supplementary Fig. 4g), in accordance with a decreased alignment of the actin cap fibers, which induces nuclear and cell stretching and flattening. Furthermore, when we analyzed the panel of actin cap assembly mediators, namely FLNA, ACTN4, SUN1 and RAC1, we recorded a significant decrease of their mRNA expression upon MET+ reintroduction (Supplementary Fig. 4h). Next, we employed Latrunculin B (LatB), an actin polymerization inhibitor that was reported4 to selectively block the formation of highly dynamic actin structures at low doses, including the actin cap filaments, while leaving the more stable basal fibers unaffected. We further proved that the actin bundles observed in KO cells were the actual perinuclear actin cap fibers. Indeed, in KO cells, LatB abolished actin caps in a time-dependent manner, as shown by phalloidin and myosin IIA staining (Supplementary Fig. 5a). Notably, also the actin-rich patches of MET+ aberrant cells seemed to be affected by LatB treatment (Supplementary Fig. 5b), confirming the initial observation of actin-based dynamically forming structures (about 12 h). In conclusion, MET+ constitutive activation induces a global misalignment and poor organization of actin cap bundles and FAs with the formation of perinuclear actin patches, while MET-KO ablation completely restores the alignment of the actin fibers network. Finally, to further validate that the morphological changes observed in terms of cell height and area in MET+ and MET-KO cells are a direct biological consequence of actin cap abrogation, we experimentally induced the actin cap disruption by a mixture of siRNAs against LINC complex components, namely Nesprin 1/2 and SUN1/2, (Supplementary Fig. 5c) in a normal epithelial model, MCF10A cells, which represent a useful tool for studying cell migration20. Here, upon siRNA transfection, we testified a complete loss of the actin cap alignment (Supplementary Fig. 5d) resulting in a mild decrease of the cell area and enhanced cell thickness (Supplementary Fig. 5e, f). Overall, these data confirm that silencing LINC complex genes, thus interfering with the actin cap, is sufficient to overcome the cell phenotype produced by loss of MET.
MET-driven perinuclear actin aberration impairs persistent cell polarization during random motility
The proper organization of the actin cap fibers is required for the establishment of directionality during persistent movements in random motility settings8. Indeed, the cap fibers align according to the direction of cell migration for relatively long periods of time (from 30’ to 2 h), fostering nucleus translocation, and then disassemble to allow nucleus rotation and re-polarization in the direction of the next persistent move8. Thus, we decided to investigate cell polarization and directionality using Phasefocus LiveCyteTM apparatus. Consistently with an aberrant actin cap organization, MET+ cells showed a random migration pattern characterized by scattered and meandering movements, often resulting in condensed tracks starting and ending at the same point on X-Y (Fig. 4a, Supplementary video 6). The migratory phenotype observed in MET+ cells suggests that the identified actin patches, by remaining dynamically coupled to the nuclear envelope through the LINC complex, impair polarized movements in place of random nuclear rotation. On the other hand, MET-KO cells showed well-polarized paths and perfectly calibrated persistent moves, resulting in linear and straight tracks (Fig. 4b, Supplementary video 7). In association with the different migratory behavior, MET expressing cells displayed remarkably increased velocity compared to the KO (Fig. 4c, d). Quantitatively, the different behavior of MET aberrant and KO cells can be described by the difference in the confinement ratio, which measures the straightness of a cell track21. Indeed, MET-KO cells displayed impressively higher confinement ratio, compatible with a directed and polarized motility, compared to the MET+ expressing cells (Fig. 4e). Interestingly, the rescue of MET hyperactivation through TPR-MET insertion in LoVo MET-KO cells was sufficient to prompt cell motility, with TPR-MET cells showing strikingly higher velocity compared to MET-KO (Fig. 4f, g, Supplementary video 8). Nonetheless, MET+ rescued cells displayed a reduced confinement ratio, confirming that MET signaling hyperactivation is per se responsible for the determination of migratory behavior (Fig. 4h, Supplementary video 8). To sum up, MET-mediated cytoskeletal alterations interfere with cell polarization during persistent random motility, by preventing the formation of a properly aligned actin cap, and most likely by favoring nuclear rotation at the expense of nuclear translocation. To confirm that these changes in cell motility were a direct consequence of the actin cap disruption, we monitored over-time the migration of normal cells (MCF10A) upon actin cap abrogation through siRNA against SUN1/2 and Nesprin 1/2. In line with our data, siRNA-treated MCF10A showed a decreased confinement ratio, although also cell velocity was reduced (Supplementary Fig. 5g, h). All together these results confirmed a driver role for MET on impairing actin cytoskeleton assembly, loss of the actin cap genes and a scattered and amoeboid motility, supporting the original hypothesis that a properly aligned actin cap may mediate polarized and straight movements.
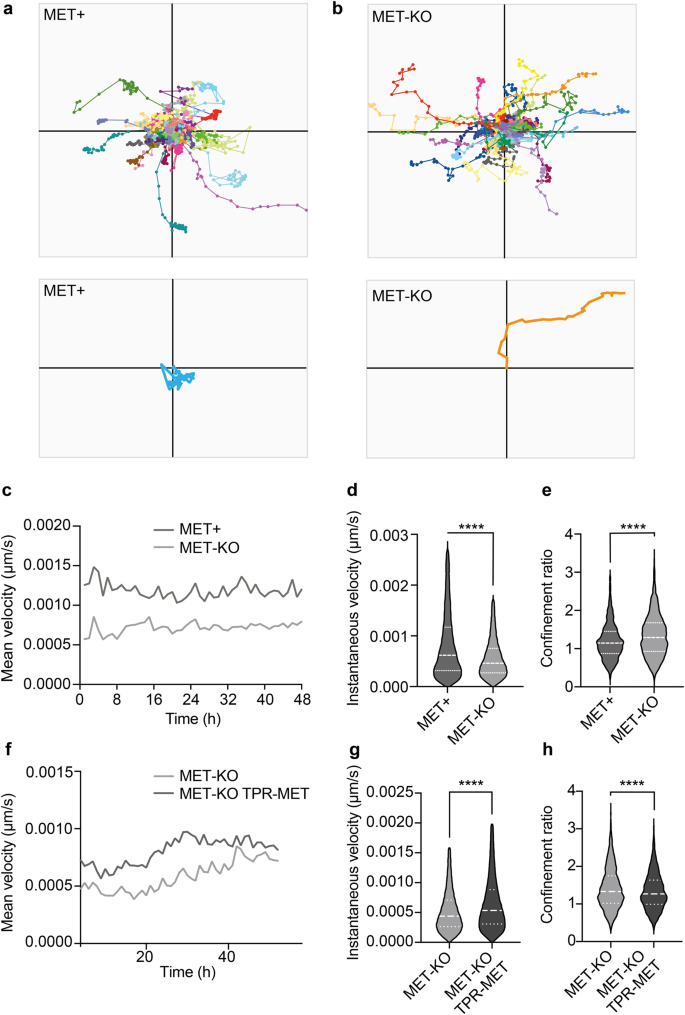
a, b Cell tracking of LoVo MET+ and MET-KO cells monitored for 48 h with Phasefocus LiveCyteTM platform. A total of 100 cells tracks are reported. Representative cell tracks were isolated and enlarged. Representative videos are reported as Supplementary videos 6, 7. c–e Charts showing mean velocity, instantaneous velocity and confinement ratio of MET+ and MET-KO cells from experiment in (a, b). Confinement ratio was computed by Phasefocus LiveCyteTM software for each time frame for a track, and data are reported as the mean of these (track averaged confinement ratio). Median and quartiles distribution are reported. 800 cells were evaluated. Statistic was calculated by T-test on cleaned data according to ROUT method (Q = 1%). f–h Mean velocity, instantaneous velocity and confinement ratio of MET-KO and MET-KO TPR-MET (rescued) cells monitored over time as in (a, b). Median and quartiles distribution are reported. Statistic was calculated by T-test on cleaned data according to ROUT method (Q = 1%). 1500 cells were analyzed.
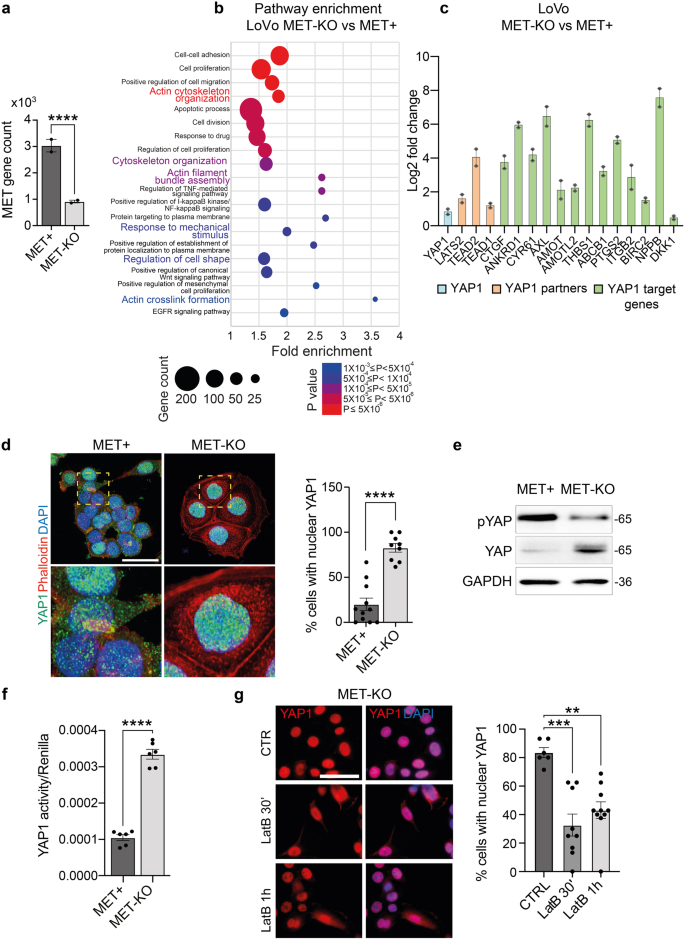
a MET (ENSG00000105976) gene count according to RNAseq data in MET+ and MET-KO cells. b Bubble chart of pathway enrichment analysis performed on RNAseq data showing the main pathways with differential expression between LoVo MET-KO vs MET+ cells. The size of the bubble correlates with gene count while the color represents statistical significance. Only pathways with P < 0.001 are shown. c Differential expression of known YAP1-related genes and YAP1-target genes in MET+ and MET-KO cells according to RNAseq data. Data are shown as Log2 fold change ± SDERR. d Evaluation of YAP1 subcellular localization in MET+ and MET-KO cells by means of YAP1 immunostaining (green). Nuclei were counterstained with DAPI (blue) and actin cytoskeleton was stained by Phalloidin (red). The quantification provides the % of cells showing a complete nuclear localization of YAP1, measured in at least 8 not overlapping fields per cell line. Data are shown as mean ± SEM. Statistic was calculated by T-test. A total of 300 cells was analyzed. Scale ‘bar: 25 μm. e Western blot analysis of YAP1 protein levels and YAP1 Ser127-phosphorylation in MET+ and MET-KO cells. GAPDH was used as loading control. f Analysis of YAP1 activity in MET+ and MET-KO cells by luciferase assay with 8xGTIIC-luciferase reporter plasmid. Renilla luciferase was used as normalizing transfection control. Data are shown as mean ± SEM and statistic was calculated by T-test. g YAP1 (red) and DAPI (blue) staining in LoVo MET-KO cells treated with 80 nM Latrunculin B for 30’ and 1 h. The quantification was performed on at least 6 fields per condition and shows the % of cells with a complete nuclear localization of YAP1. Data are reported as mean ± SEM. Statistic was calculated by One-way ANOVA. A total of 300 cells were analyzed. Scale bar: 50 μm.
MET activation controls YAP1 nuclear export and transcriptional activity
Aiming to characterize the genetic profile driving these striking morphological changes, we performed RNA sequencing analysis in MET+ and KO cells. Interestingly, MET was among the most significantly downregulated genes in MET-KO cells, indicating an impaired stability of the mRNA induced by the CRISPR/Cas9 point mutation (Fig. 5a). The pathway enrichment analysis revealed an involvement of several signaling cascades, including those responsible in actin cytoskeleton organization, such as actin filament bundle assembly and actin crosslink formation, as well as regulation of the cell shape. Intriguingly, also the biological processes entangling cell response to mechanical stimuli appeared to be differentially expressed between the two models (Fig. 5b). Prompted by these results, we analyzed the involvement of YAP1, which transduces mechanical cues into gene expression programs22. Strikingly, a wide set of YAP1-regulated transcriptional targets, including the genes CYR61, ANKRD1, THBS1, PTGS2, CTGF, NPPB and AXL, appeared robustly upregulated in MET-KO cells compared to MET+ cells (Fig. 5c). Thus, we tested YAP1 activation by evaluation of subcellular localization. YAP1 was shown to localize primarily in the nucleus in MET-KO cells, while having a mixed diffuse cytosolic/nuclear localization in the MET+ cells, indicating a lower activation of the pathway due to cytoplasmic shuttling (Fig. 5d). Accordingly, YAP1 total form was decreased in protein lysates from aberrant MET+ compared to KO cells, compatibly with higher levels of YAP1 degradation following its relocation to the cytoplasm (Fig. 5e). Interestingly, the levels of YAP1 inhibitory phosphorylation on Ser127, resulting from LATS kinases cascade activation upon Hippo pathway engagement, were shown to be only slightly decreased in MET-KO model (Fig. 5e). In line, experiments with a luciferase reporter for YAP1 activity (8xGTIIC-Lux) showed a massive increment of YAP1 activation in basal conditions in KO cells compared to the MET+ cells (Fig. 5f). Following the strong inactivation of YAP1 observed in LoVo cells, we decided to evaluate YAP1 localization and protein levels also in an additional MET-dependent gastric carcinoma model, GTL16. GTL16 cells showed a massive protein expression and high phosphorylation levels of MET, while YAP1 was barely detectable (Supplementary Fig. 2d) most likely due to an increased trafficking to the cytoplasm (Supplementary Fig. 2e), where YAP1 degradation occurs. In addition, MET rescue in LoVo MET-KO cells by introduction of TPR-MET was sufficient per se to hamper YAP1 signaling by inducing YAP1 cytosolic relocation (Supplementary Fig. 4i). As a result, several YAP1 target genes were highly downregulated in rescued cells, mimicking YAP1 status before MET silencing (Supplementary Fig. 4j). Collectively these data suggest that MET hyperactivation is sufficient to induce YAP1 cytosolic localization, thus impairing its co-transcriptional activity. We further proved that upon MET ablation, cells experience strong dependency on YAP nuclear localization. Indeed, when forcing YAP to re-localize to the cytoplasm by changing the stiffness of the substrates and employing a soft ECM, namely matrigel or agar, MET-KO cells completely lost the capability to grow (Supplementary Fig. 6a–d, Supplementary videos 9, 10). Finally, impairment of the perinuclear actin cap by administration of low doses of LatB in KO cells, was sufficient to induce YAP1 cytoplasmic translocation (Fig. 5g). To sum up, we confirmed that MET ablation induces subcellular nuclear localization of YAP1, thus modulating its activity and the production of genes responsible for the perinuclear actin cap formation23,24. Next, an exogenous form of YAP harboring five serines-to-alanines mutations (5SA) that prevent the inhibition and degradation induced by phosphorylation, was inserted in LoVo and GTL16 cells and confirmed by western blot (Fig. 6a). Notably, YAP activation finely reproduced the MET silencing, being particularly evident in GTL16 cells, where marked differences in the cell phenotype are already visible with optical microscopy (Fig. 6b). The proliferation rate of both LoVo and GTL16 cells was found to be reduced by YAP5SA insertion, supporting the model that YAP could hold an anti-proliferative role in gastrointestinal cancer cells (Fig. 6c). Live-cell imaging assays revealed a significant increase in cell area (Fig. 6d), perimeter (Fig. 6e), but a decrease in thickness (Fig. 6f) and in the sphericity (Fig. 6g) in both GTL16 and LoVo cells. Moreover, cell velocity was found to be statistically decreased in both cell lines following the introduction of YAP5SA (Fig. 6h). To sum up, the constitutive activation of nuclear YAP blunted the main effects of oncogenic MET. Nonetheless, further investigations will be required to elucidate the mechanism by which MET/YAP regulates the preferential migratory pattern of these cells. Then we asked whether the introduction of constitutively active YAP1 was also sufficient to induce a correct alignment of the actin cap fibers, that are aberrant in LoVo and GTL16. The perinuclear actin cap was detected in both LoVo and GTL16 YAP5SA, as visualized through Phalloidin and Myosin IIA staining in the apical frame (Fig. 6i, details). In line, a mild although not statistic increase in FLNA and SUN1 expression was detected in YAP5SA expressing cells (Fig. 6j). Due to the alignment of the actin cap and the resulting nuclear flattening, nuclear height was markedly reduced in YAP5SA-expressing cells (Fig. 6k, l). Altogether, YAP5SA introduction in MET+ hyperactivated cells was shown to reproduce the phenotype of MET-KO cells, allowing to restore the actin cap fibers aberrations induced by MET. These data suggest a causative role for YAP1 in the organization of the perinuclear actin cap, supporting the model that YAP inhibition could be a direct downstream effect of MET signaling activation in gastrointestinal cancer cells.
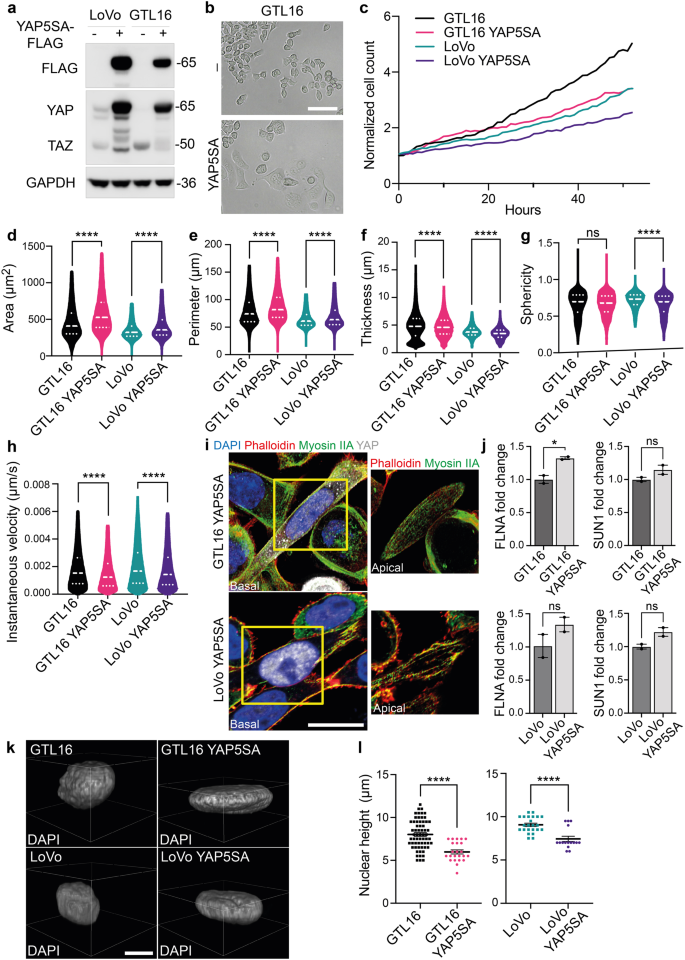
a Western blot analysis to validate YAP5SA insertion in LoVo and GTL16 cells. GAPDH was used as loading control. b Micrographs of WT and YAP5SA GTL16 cells. Scale bar: 50 μm. c Normalized cell count of LoVo and GTL16 cells infected with YAP5SA containing vector in a live-cell imaging experiment performed with Phasefocus LivecyteTM. Cells were monitored for more than 48 h and pictures were taken every hour. d–h Area, perimeter, thickness, sphericity and instantaneous velocity of LoVo and GTL16 cells WT and YAP5SA calculated from live-cell imaging in (c). Median and quartiles distribution are plotted. Outliers were identified and cleaned from results by means of ROUT method (Q = 1%). 5100 cells were analyzed. Statistic was calculated by T-test. i Confocal imaging of LoVo and GTL16 YAP5SA stained with DAPI (blue), Phalloidin (red), Myosin IIA (green) and YAP antibody (grey). A detail of the apical plane of section of cells is reported on the right. Scale bar: 10 μm. j FLNA and SUN1 expression analysis by qPCR in GTL16 and LoVo YAP5SA expressing cells. B2M was used as housekeeping gene for normalization. T-test was used for statistical analysis. k, l Analysis of nuclear height in YAP5SA expressing cells. Representative images are reported in (k), while quantification is showed in (l) and was performed as described in Fig. 2D. 120 cells were analyzed. Statistic was calculated by T-test. Scale bar: 10 μm. The scale bar refers to the xy-plane.
MET activation in normal cells drives perinuclear actin rearrangement and YAP1 deactivation
Finally, we engineered the quasi-normal epithelial cell line MCF10A, that displays a regular alignment of the apical stress fibers (Supplementary Fig. 7a), with the constitutively active fusion protein of MET, TPR-MET19. TPR-MET guarantees that MET signaling is constitutively activated, even in absence of HGF ligand administration and the correct introduction in MCF10A cells was validated by immunofluorescence (Supplementary Fig. 7b) and Western Blot (Fig. 7a). Interestingly a boosted downstream activation of the MAPK was also detected (Fig. 7a). In addition, the stimulation of MET axis caused by TPR-MET insertion was per se sufficient to reduce YAP1 total protein levels and to slightly enhance YAP1 phosphorylation on Ser127 (Fig. 7a). Consistently, in cells stably expressing TPR-MET, immunofluorescence analysis revealed a cytosolic shuttling of YAP1 in contrast with the nuclear localization detected in MCF10A reference cells (Fig. 7b). Notably, also YAP1 activity appeared halted in TPR-MET cells, which display a downregulation of YAP1 target genes PTGS2 and THBS1 (Fig. 7c). Moreover, by activating MET axis with HGF in MCF10A cells, we recorded an increased YAP1 degradation along with a decreased nuclear subcellular localization (Supplementary Fig. 7c, d). In line, MET interception by the kinase inhibitor capmatinib (CAP) in MET+ cells induced an increase in YAP1 protein abundance, although the role of MET kinase activity in the switch from the actin cap to the actin patches remain to be addressed (Supplementary Fig. 7e). Next, Transmission Electron Microscopy (TEM) was used to visualize the cytoskeleton components in MET+ constitutively activated MCF10A in comparison with naïve cells. Interestingly, MCF10A presented a neat pattern of repeated perinuclear sites, with an ordered spatial organization of about 400 nm apart, probably representing the indentation marks of the actin filament fibers covering the nuclear envelope and forming the actin cap (Fig. 7d). The introduction of aberrant MET altered this organization, the neat pattern of indentation marks disappeared by leaving concentrical aggregates far away from the nuclear envelope along with an irregular nuclear shape (Fig. 7d). Moreover, the actin cap-related genes SUN1, RAC1 and ACTN4 were found to be markedly decreased in MCF10A-TPR-MET compared to parental cells (Fig. 7e). Finally, we further proved that loss of perinuclear apical actin stress fibers in MCF10A-TPR-MET (Fig. 7f) associated with a pronounced nuclear height, probably resulting from the deficit of actin cap-mediated vertical confinement (Fig. 7g).
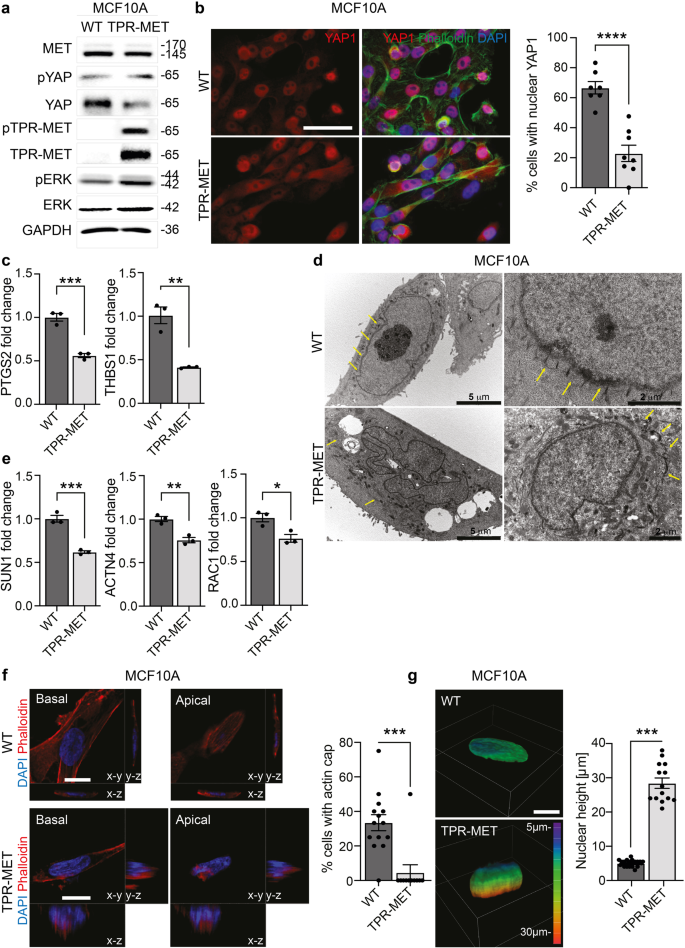
a Evaluation of YAP1 protein levels and phosphorylation on Ser127 in MCF10A WT and TPR-MET cells. The introduction of constitutively phosphorylated 65 kDa TPR-MET in MCF10A is confirmed together with downstream MAPK signaling activation. b Evaluation of YAP1 subcellular localization in MCF10A WT and TPR-MET cells. Cells were counterstained with Phalloidin (green) and DAPI (blue). The % of cells with complete nuclear YAP1 is reported in the quantification on the right. At least 7 independent fields were analyzed and the quantification relies on a total of 158 cells. Data are shown as mean ± SEM. Statistic was calculated by T-test. Scale bar: 50 μm. c Analysis of YAP1 target genes PTGS2 and THBS1 RNA expression by qPCR in MCF10A WT and TPR-MET. Data are shown as fold change in relation to MCF10A WT ± SD. Unpaired T-test was applied for statistical analysis. d Transmission electron microscopy of MCF10A WT and TPR-MET. Yellow arrows point at actin cap filaments. Individual scale bar is indicated for each image. e Determination of and actin cap-related genes SUN1, ACTN4 and RAC1 expression by qPCR in MCF10A WT and TPR-MET. Data are reported as fold change in relation to MCF10A WT ± SD. Statistic was calculated by T-test. f Analysis of actin cap presence in the apical xy-plane of MCF10A WT and TPR-MET by means of Phalloidin (red) and DAPI (blue) staining. yz and xz Z-stacks are also reported. The quantification shows the % of cells with properly assembled actin cap, as measured in at least 11 fields per cell line. For MCF10A TPR-MET, only cells highly positive to pMET cytosolic staining were analyzed. A total of 100 cells were analyzed. Statistic was calculated by T-test. Scale bar: 10 μm. g Assessment of nuclear height in MCF10A WT and TPR-MET from experiment in (d). Quantification was performed by analyzing Z-stacks images of 40 cells. Representative 3D rendering images are provided, and Z-depth coding is reported as a scale of colors. Statistic was calculated by T-test. Scale bar: 10 μm. The scale bar refers to the xy-plane.
[ad_2]
Source link
